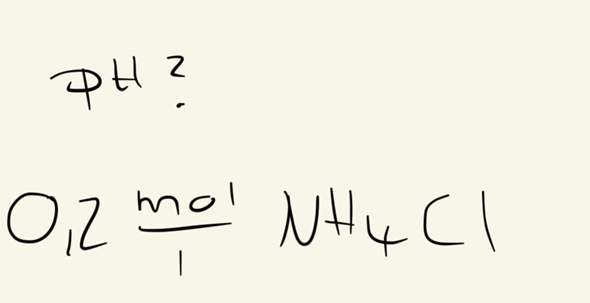Acid-sensitive ionic channels in midbrain dopamine neurons are sensitive to ammonium, which may contribute to hyperammonemia damage | PNAS

Total Synthesis of (−)-Agelastatin A: The Application of a Sequential Sigmatropic Rearrangement | Organic Letters

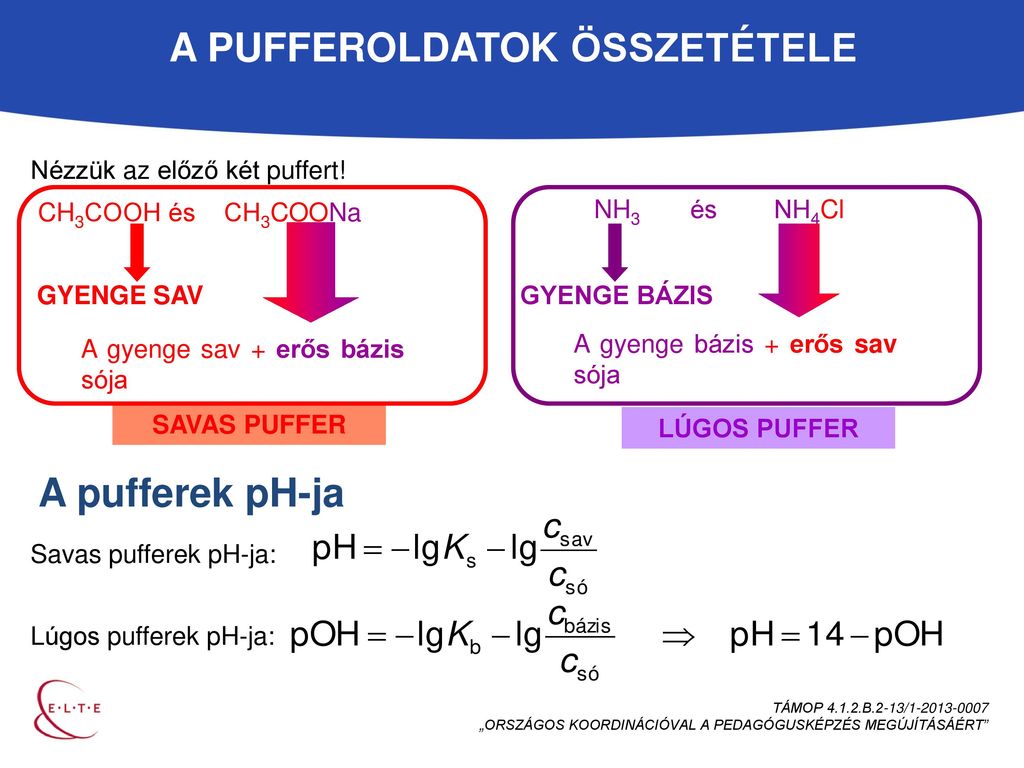
Calculate the pH of a buffer prepared by mixing 300 cc of 0.3 M NH3 and 500 cc of 0.5 M NH4Cl . Kb for NH3 = 1.8 × 10^-5

Acid-sensitive ionic channels in midbrain dopamine neurons are sensitive to ammonium, which may contribute to hyperammonemia damage. - Abstract - Europe PMC

Seminaraufgaben - Übungen zur Vorlesung Anorganische Experimentalchemie (AC1 im WS) mit Lösungen. - Studocu

Acid-sensitive ionic channels in midbrain dopamine neurons are sensitive to ammonium, which may contribute to hyperammonemia damage | PNAS
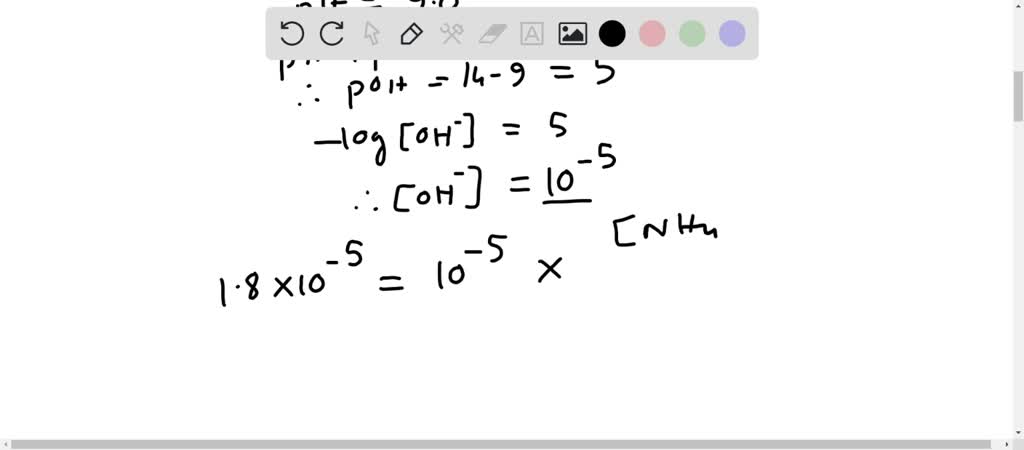
SOLVED: How many moles of NH4Cl must be added to 2.0 L of 0.10M NH3 to form a buffer whose pH is 9.00? (Assume that the addition of NH4Cl does not change


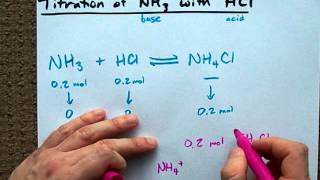
![Pufferlösung, pH 10-11 [NH4Cl/NH4OH], BAKER ANALYZED , J.T. Baker | Fisher Scientific Pufferlösung, pH 10-11 [NH4Cl/NH4OH], BAKER ANALYZED , J.T. Baker | Fisher Scientific](https://assets.fishersci.com/TFS-Assets/CCG/product-images/default.jpg-650.jpg)